SSC English Version Chemistry Assignment Answer 2021: Directorate of Secondary and Higher Secondary Education is published the SSC English Version Chemistry Assignment 2021 in pdf file on dshe.gov.bd. website.
DSHE is published the 1st Week Chemistry English Version assignment for the students of science of English version.
SSC English Version Chemistry Assignment Answer 2021
DSHE is published the SSC English Version Chemistry Assignment Question on the official website. Students now can check the assignment question on dshe.gov.bd.
Assignment No: 1
Assignment Number, Chapter Number, Chapter Title- Chapter Three: Structure of Matter
Assigned Work:
Number of neutrons in four different elements, Figure of structure of atom according to the Bhor’s model, Electronic configuration of energy level and sub-energy level(orbitals) Prepare a report on the number of neutrons in the mass numbers of the elements mentioned next to the symbols, the diagram of the structure of atoms according to Bohr model, their electronic configuration at energy levels and sub- energy (orbitals) levels. Na(11), Mass number-23 P(15), Mass number-31 K(19), Mass number-40 Cu(29), Mass number-63
Learning Outcomes:
- Able to determine the number of electrons, protons and neutrons in an atom
- Able to describe the structure of atom in elation to the theories of Rutherford and Bohr atomic model
- Able to write the electronic configurations of different orbits and different sub levels of orbits of an atom.
Guidelines clues/steps or stages
- Has to find out the number of neutrons in our elements
- Has to draw he figure of structure of tom according o the Bhor‟s model
- Has to write he electronic configurations of energy level of four elements
- Has to write he electronic configurations of sub-energy level (orbitals) of four elements

Assignment No: 2
Assignment Number, Chapter Number, Chapter Title- Chapter Four: Periodic Table
Assigned Work:
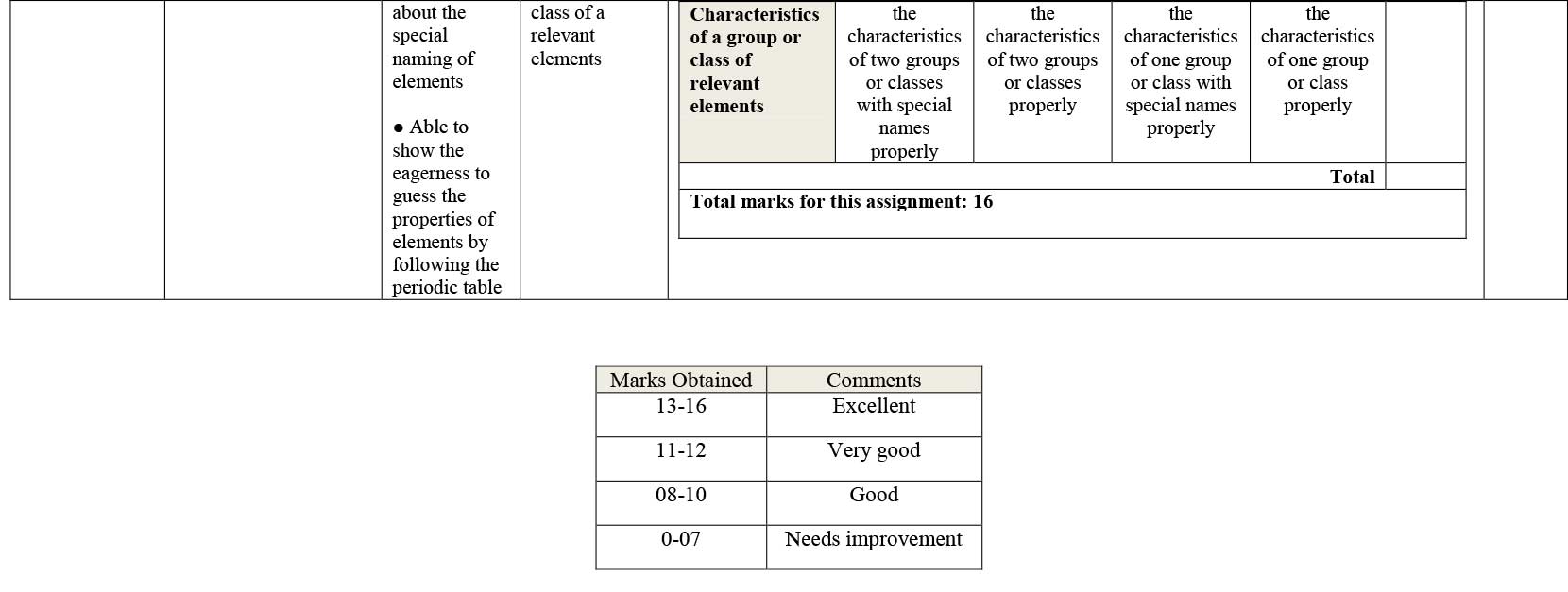
Assignment Position of the elements in the periodic table according to the electronic configuration, Comparative ionization energy and Characteristics of group or class of relevant elements:
Li Be Na Mg
In accordance to the electronic configuration of the 4 elements, prepare a report on their position in the periodic table, comparative ionization energy and the characteristics of the groups they are in.
Learning Outcomes:
- Able to determine the relation of major groups of periodic table with the outer most energy level electronic configurations of elements(first 30 elements)
- Able to find out the period of an element
- Able to get the knowledge about physical and chemical properties of an element by knowing the position in the periodic table
- Able to say about the special naming of elements
- Able to show the eagerness to guess the properties of elements by following the periodic table.
Guidelines (cues/steps or stages)
- Has to find out the periods of the four elements in the periodic table according to the electronic configurations
- Has to find out the groups or classes of the four elements in the periodic table according to their electronic configurations
- Has to compare the ionization energy of same period and same group or class of adjoining elements in the periodic table
- Has to write the characteristics of the group or class of a relevant elements

Source: DSHE